Transformation to A Strong Compliance Culture
 1Anybody leading an organization in a new direction has a tough job. Our company, Validation & Compliance Institute (VCI), was tasked with helping a site of several hundred employees to dramatically improve compliance with FDA regulations. This was an especially difficult project, considering that, like most biotech organizations, the company leaders were technical managers. People skills were not their strong suit, at least, not at the beginning…
1Anybody leading an organization in a new direction has a tough job. Our company, Validation & Compliance Institute (VCI), was tasked with helping a site of several hundred employees to dramatically improve compliance with FDA regulations. This was an especially difficult project, considering that, like most biotech organizations, the company leaders were technical managers. People skills were not their strong suit, at least, not at the beginning…
Last time I talked about Conrad Hilton's secret to business success, that you need to have systems which connect the dots between your strategies and the people with their hands on the work. This article is a brief look at the cultural change that was accomplished in this Michigan organization. At the end of the implementation phase the site had radically improved its quality performance, AND the client had the numbers to prove it.
As with many companies that are subject to Food and Drug Administration regulations, our client had spent a lot of time writing procedures and training their employees how to comply with the regulations. Their problem was not a lack of commitment. They had plenty of resources assigned to the project. Their problem was not a lack of knowledge. They had talented regulatory specialists and line managers devoted to improving the company’s compliance performance.
No, rather their problem was in translating management’s goals, resources, and knowledge into daily results on the factory and laboratory floor. Not that they did a bad job, mind you; far from it. In fact, if you tracked their batting average, it was in excess of 99%. The problem is that making a batch of a drug right is a very complicated process. From raw materials, through manufacturing, analysis, and paperwork there are usually more than 10,000 individual tasks that need to be done right. 99% right-the-first-time-leaves you with 100 errors.
Our client was spending too much precious time and resources correcting those errors. They were sending too many people for retraining. They had so many Corrective and Preventive Actions (CAPAs) they couldn’t keep track of them all. They needed to find a way to reduce costs and be more compliant at the same time.
Exhortations from executives to managers to watch their employees more closely only resulted in their inboxes piling higher. Intensive pep talks to employees to be more careful only caused a veil of secrecy to be drawn around the errors.
The client had tried, unsuccessfully, all the conventional top-down methods to improve employee performance. They needed an alternate strategy.
VCI introduced the client to Positive ComplianceTM, a system that turned their weaknesses into assets. The analytical skills of their managers, which had oftentimes come between management and employees, became the tool that enabled the company to improve performance.
We took advantage of the work of Frederick Herzberg2 in designing Positive ComplianceTM. It allows companies to focus employee teams on the sources of errors in their processes. Herzberg’s research shows that the two most important factors that motivate employees are achievement and recognition. Positive ComplianceTM uses the power of Herzberg’s motivational research and the guidance of measurements to focus employee teams on business critical goals.
The first major target area was batch record completion accuracy. The employees were grouped into their regular work teams (production shifts, shipping/receiving, laboratory, Process Development, Engineering, etc). Each team was asked to think of something that they did that affected to batch record accuracy. They were then asked to develop SMART goals – Specific, Measurable, Attainable, Relevant, Time-bound – that, if achieved, would result in improved batch record accuracy. The fact that the goals were team based encouraged the employees to help each other to achieve the goals.
The critical aspect of the goal setting process was that the goals for each team had to be something that the individual team had control over. Clearly, some teams had more direct impact on batch record completion accuracy than others. And this was a point of some questioning initially. But once all the inputs that are necessary in order to accurately complete a batch record were fully laid out, it became clear how integrated the process was throughout the company.
For instance, Process Development was responsible for initial batch record design. Their contribution to the improvement process was to modify the format of the batch records based on the input of operations.
Some of the managers struggled when the time came to free the teams to work toward achieving the goals in their own way. With repeated achievements, though, the managers learned how to provide enough room for the teams to find their own way to success. They discovered that the teams felt more ownership of the results, and were more likely to comply with the procedures that they, themselves, had shaped.
As the teams progressed toward fulfilling their goals, they charted their improvement. The charts were posted in visible locations, thus providing proof of their achievements, one of Herzberg’s true employee motivators. Management invested small amounts of money in awards for teams as they reached their goals. More importantly, however, they showed up in person to recognize the achievements of the teams.
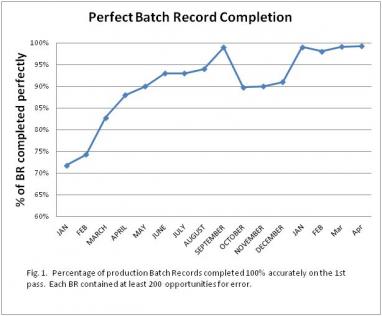 Gradually the GDP error rate dropped. We posted the chart in a visible location and that provided positive feedback to our efforts (Fig 1).
Gradually the GDP error rate dropped. We posted the chart in a visible location and that provided positive feedback to our efforts (Fig 1).
After they completed the batch record project, the teams moved on to others and repeated the process. These were not big picture projects. Rather they were small, like beard cover compliance. Nevertheless, these are the kinds of details that can make or break a company if they are not functioning near 100% compliance.
Positive ComplianceTM is not a get rich quick scheme. But substantial dollar savings resulted from the reduction in time spent correcting batch records. We had estimated that it took five work-hours to get a batch record corrected; and it took two weeks of calendar time. This meant, of course, a two week delay in shipping, two weeks of dead inventory!
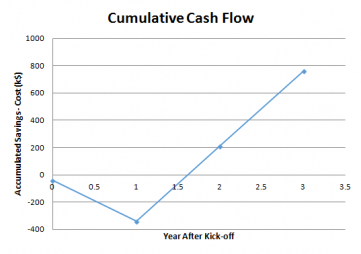 The time consumed by Positive ComplianceTM is minimal, about an hour per employee per week. Constructive results began to appear after several weeks. Within a year and a half the hemorrhaging had stopped (Fig. 2). The savings continued to roll in going forward. The client’s exposure to FDA enforcement had fallen substantially. AND the client had the measurements to prove it!
The time consumed by Positive ComplianceTM is minimal, about an hour per employee per week. Constructive results began to appear after several weeks. Within a year and a half the hemorrhaging had stopped (Fig. 2). The savings continued to roll in going forward. The client’s exposure to FDA enforcement had fallen substantially. AND the client had the measurements to prove it!
In addition, the client reaped the following benefits:
- The teams learned how to measure simple employee behaviors
- Everyone learned how to find the performance inputs in their jobs that affect business critical outcomes
- Managers learned to be better delegators
- Managers learned how to turn their analytical mindset into an asset when interacting with people
- Managers learned how to better motivate employees
- Employees became more willing to help each-other
- Managers AND workers together drove FEAR out of the organization
But possibly the most satisfying outcome for the client managers was the experience of their employees taking responsibility for the improvement of daily operations.
Ok, I want to find out more. What should I do?
Call 734-274-4680 or Ask VCI to help you in creating a quality culture.
1This was originally published in BioMatters, Fall 2013.
2One More Time: How Do You Motivate Employees? Frederick Herzberg, Harvard Business Review, September 2003
How about you? Do you have a similar story? What has worked best for you? Comment below.

Comments
Good website,
Good website,
To the vcillc.com admin, Your
To the vcillc.com admin, Your posts are always well-formatted and easy to read.
Thank you, Permalink!
Thank you, Jose!
Add new comment